2023
84. Kamlar. M.; Urban, M.; Veselý, J. "Enantioselective Synthesis of Spiro Heterocyclic Compounds Using a Combination of Organocatalysis and Transition-Metal Catalysis" Chem.Rec. 2023, e202200284.
https://doi.org/10.1002/tcr.202200284

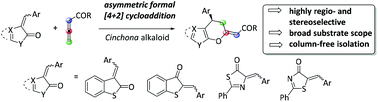
83. Lóška, L.; Dočekal, V.; Císařová, I.; Veselý, J. "Stereoselective N-Heterocyclic-Carbene-Catalyzed Formal [4 + 2] Cycloaddition: Access to Chiral Heterocyclic Cyclohexenones" Org. Lett. 2023, 25, 174–178.
https://doi.org/10.1021/acs.orglett.2c04021

2022
82. Kohoutova, K.; Dočekal, V.; Ausserlechner, M. J.; Kaiser, N.; Tekel, A.; Mandal, R.; Horvath, M.; Obsilova, V.; Veselý, J.; Hagenbuchner, J.; Obsil, T. "Lengthening the Guanidine−Aryl Linker of Phenylpyrimidinylguanidines Increases Their Potency as Inhibitors of FOXO3-Induced Gene Transcription" ACS Omega, 2022, 7, 34632−34646.
https://doi.org/10.1021/acsomega.2c04613

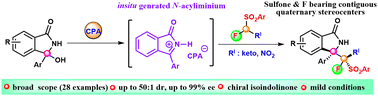
81. Bhosale, V. A.; Císařová, I.; Kamlar, M.; Veselý, J. "Catalytic asymmetric addition to cyclic N-acyl-iminium: access to sulfone-bearing contiguous quaternary stereocenters" Chem. Commun., 2022, 58, 9942–9945.
https://doi.org/10.1039/D2CC02667H
80. Urban, M.; Veselý, J.: Enantioselective Synthesis of Spiro Heterocycles. In: Spiro Compounds: Synthesis and Applications, R. Rios (ed.), Wiley, VCH, 2022. ISBN: 978-1-119-567-639
https://doi.org/10.1002/9781119567646.ch7
Chapter in the book
2021
79. Dočekal, V.; Koberová, T.; Hrabovský, J.; Vopálenská, A.; Gyepes, R.; Císařová, I.; Rios, R.; Veselý, J. Adv. Synth. Catal. 2021, 364, 930-937.
VIP, Cover Art, Hot Topic: Organocatalysis
https://doi.org/10.1002/adsc.202101286

78. Bhosale, V. A..; Nigríni, M.; Dračínský, M.; Císařová, I.; Veselý, J. "Enantioselective Desymmetrization of 3-Substituted Oxetanes: An Efficient Access to Chiral 3,4-Dihydro-2H-1,4-benzoxazines." Org. Lett. 2021, 23, 9376-9381.
https://doi.org/10.1021/acs.orglett.1c03419

77. Urban, M.; Nigríni, M.; Císařová, I.; Veselý, J. "Enantioselective Construction of Chiral Bispiro[Oxindole-Pyrrolidine-Pyrazolone] Derivatives via Sequential and One-Pot Mannich/Hydroamination Reaction." J. Org. Chem. 2021, 86, 18139–18155.
https://doi.org/10.1021/acs.joc.1c02428

76. Kamlar, M.; Reiberger, R.; Nigríni, M.; Císařová, I.; Veselý, J. " Enantioselective PCCP Bronsted Acid-catalyzed Aminalization of Aldehydes." Beilstein J. Org. Chem. 2021, 17, 2433-2440.
https://doi.org/10.3762/bjoc.17.160
75. Franc, M.; Císařová,I.; Veselý, J. "Enantioselective Synthesis of Spirothiazolones via Cooperative Catalysis" Adv. Synth. Catal. 2021, 363, 4349-4353.
https://doi.org/10.1002/adsc.202100571

74. Šotolová, M.; Kamlar, M.; Remeš, M.; Geánt, P.-Y.; Císařová, I.; Štícha, M.; Veselý, J. "Enantioselective Organocatalytic Synthesis of 1,2,3-Trisubstituted Cyclopentanes" Eur. J. Org. Chem. 2021, 5080-5089.
https://doi.org/10.1002/ejoc.202100841

73. Dočekal, V.; Vopálenská, A.; Měrka, P.; Konečná, K.; Janďourek, O.; Pour, M.; Císařová, I. and Veselý, J. "Enantioselective Construction of Spirooxindole-Fused Cyclopentanes" J. Org. Chem. 2021, 18, 12623–12643.
https://doi.org/10.1021/acs.joc.1c01116

72. Formánek, B.;Šeferna, V.; Meazza, M.; Rios, R.; Patil, M. and Veselý, J. "Organocatalytic Amination of Pyrazolones with Azodicarboxylates: Scope and Limitations" Eur. J. Org. Chem. 2021, 2362–2366.
https://doi.org/10.1002/ejoc.202100167
2020
71. Formánek, B.; Tauchman, J.; Císařová, I. and Veselý, J. "Access to Spirocyclic Benzothiophenones with Multiple Stereocenters via an Organocatalytic Cascade Reaction" J. Org. Chem. 2020, 85, 8510−8521.
https://doi.org/10.1021/acs.joc.0c00882

70. Dočekal, V.; Petrželová, S.; Císařová, I. and Veselý, J. "Enantioselective Cyclopropanation of 4-Nitroisoxazole Derivatives" Adv. Synth. Catal. 2020, 362, 2597– 2603.
https://doi.org/10.1002/adsc.202000231

2019
69. Hagenbuchner, J.; Obšilová, V.; Kaserer, T.; Kaiser, N.; Rass, B.; Pšenáková, K.; Dočekal, V.; Alblová, M.; Kohoutová, K.; Schuster, D.; Aneichyk, T.; Veselý, J.; Obexer, P.; Obšil, T.; Ausserlechner, M. J. "Modulating FOXO3 transcriptional activity by small, DBD-binding molecules." eLife 2019, 8, e48876.
DOI: 10.7554/eLife.48876
68. Formánek, B.; Šimek, M.; Kamlar, M.; Císařová, I. and Veselý, J. "Organocatalytic Allylic Amination of Morita–Baylis–Hillman Carbonates" Synthesis 2019, 51, 907-920.
https://doi.org/10.1055/s-0037-1611229

67. Dočekal, D.; Formánek, B.; Císařová, I. and Veselý, J. "A formal [4 + 2] cycloaddition of sulfur-containing alkylidene heterocycles with allenic compounds" Org. Chem. Front. 2019, 6, 3259–3263.
https://doi.org/10.1039/C9QO00886A
66. Kamlar, M.; Franc, M.; Císařová, I.;Gyepes, R. andVeselý, J. "Formal [3+2] cycloaddition of vinylcyclopropane azlactones to enals using synergistic catalysis" Chem. Commun. 2019, 55, 3829-3832.
https://doi.org/10.1039/C8CC06500D
65. Franc, M.; Urban, M.; Císařová, I. and Veselý, J. "Highly enantioselective addition of sulfurcontaining heterocycles to isatin-derived ketimines" Org. Biomol. Chem. 2019, 17, 7309.
https://doi.org/10.1039/C9OB01338E
64. Putatunda, S.; Alegre-Requena, J. V. Meazza, M.; Franc, M.; Rohal'ová, D.; Vemuri, P.; Císařová, D.; Herrera, R. P.; Rios R. and Veselý, J. "Proline bulky substituents consecutively act as steric hindrances and directing groups in a Michael/Conia-ene cascade reaction under synergistic catalysis" Chem. Sci. 2019, 10, 4107–4115.
https://doi.org/10.1039/C8SC05258A
2018
63. Gergelitsová, I.; Veselý, J.: Diastereoselektive addition of organometallic reagents to chiral carbonyl compounds. In Asymmetric Synthesis of Drugs and Natural Products, A. Nag (ed.), Taylor & Francis, CRC Press, Boca Raton, 2018. ISBN:978-1-138-03361-0.
Chapter in the book
62. Dočekal, V.; Šimek, M.; Dračínský, M. and Veselý, J. "Decarboxylative Organocatalytic Allylic Amination of Morita–Baylis–Hillman Carbamates" Chem. Eur. J. 2018, 24,13441 –13445.
https://doi.org/10.1002/chem.201803677

61. Meazza, M.; Kamlar, M.; Jašíková, L.; Formánek, B.; Mazzanti, A.; Roithová, J.; Veselý, J. and Rios, R. "Synergistic formal ring contraction for the enantioselective synthesis of spiropyrazolones" Chem. Sci. 2018, 9, 6368–6373.
https://doi.org/10.1039/C8SC00913A
2017
60. Kamlar, M.; Císařová, I.; Hybelbauerová, S. and Veselý, J. "Asymmetric Allylic Amination of Morita–Baylis–Hillman Carbonates with Silylated tert-Butylhydroxycarbamate Derivatives" Eur. J. Org. Chem. 2017, 1926–1930.
https://doi.org/10.1002/ejoc.201700222

59. Zhang, K.; Meazza, M.; Dočekal, V.; Light, M. E.; Veselý, J. and Rios, R. "Highly Diastereo- and Enantioselective Synthesis of α-Spiro-δ-lactams by an Organocascade Reaction" Eur. J. Org. Chem. 2017, 1749–1756.
ttps://doi.org/10.1002/ejoc.201700193

58. Urban, M.; Franc, M.; Hofmanová, M.; Císařová, I. and Veselý, J. "The enantioselective addition of 1-fluoro-1-nitro(phenylsulfonyl)methane to isatin-derived ketimines" Org. Biomol. Chem. 2017, 15, 9071–9076.
https://doi.org/10.1039/C7OB02408H
2016
57. Cousido-Siah, A.;Ruiz, F. X.;Fanfrlík,J.; Gimenez-Dejoz, J.; Mitschler, A.; Kamlar, M.; Veselý J.; Ajani,H.; Pareś, X.; Farreś, J.; Hobza, P. and Podjarny, A. D. "IDD388 Polyhalogenated Derivatives as Probes for an Improved Structure-Based Selectivity of AKR1B10 Inhibitors" ACS Chem. Biol. 2016, 11, 2693–2705.
https://doi.org/10.1021/acschembio.6b00382

56. Humpl, M.; Tauchman, J.; Topolovčan, N.; Kretschmer, J.; Hessler, F.; Císařová, I.; Kotora, M.; and Veselý, J. "Stereoselective Synthesis of Ezetimibe via Cross-Metathesis of Homoallylalcohols and α-Methylidene-β-Lactams" J. Org. Chem. 2016, 81, 7692–7699.
https://doi.org/10.1021/acs.joc.6b01406

2015
55. Kamlar, M.; Císařová, I. and Veselý, J. "Alkynylation of heterocyclic compounds using hypervalent iodine reagent" Org. Biomol. Chem. 2015, 13, 2884–2889.
https://doi.org/10.1039/C4OB02625J
54. Gergelitsová, I.; Tauchman, J.; Císařová, I. and Veselý, J. "Bifunctional (Thio)urea–Phosphine Organocatalysts Derived from d-Glucose and α-Amino Acids and Their Application to the Enantioselective Morita–Baylis–Hillman Reaction" Synlett 2015, 26, 2690-2696.
https://doi.org/10.1055/s-0035-1560931

53. Ceban, V.; Tauchman, J.; Meazza, M.; Gallagher, G.; Light, M. E.; Gergelitsová, I.; Veselý, J. and Rios, R. "Expanding the scope of Metal-Free enantioselective allylic substitutions: Anthrones"
https://doi.org/10.1038/srep16886 (2015).
2014
52. Veselý, J. and Rios, R. "Enantioselective methodologies using N-carbamoyl-imines" Chem. Soc. Rev. 2014, 43, 611-630.
https://doi.org/10.1039/C3CS60321K
51. Putaj, P.; Tichá, I.; Císařová, I. and Veselý, J. "One-Pot Preparation of Chiral Carbacycles from Morita–Baylis–Hillman Carbonates by an Asymmetric Allylic Alkylation/Olefin Metathesis Sequence" Eur. J. Org. Chem. 2014, 6615–6620.
https://doi.org/10.1002/ejoc.201402899

50. Kamlar, M.; Hybelbauerová, S.; Císařová, I. and Veselý, J. "Organocatalytic enantioselective allylic alkylation of MBH carbonates with β-keto esters" Org. Biomol. Chem. 2014, 12, 5071–5076.
https://doi.org/10.1039/C4OB00682H
49. Tsybizova, A.; Remeš, M.; Veselý, J.; Hybelbauerová, S. and Roithová, J. "Organocatalytic Preparation of Substituted Cyclopentanes: A Mechanistic Study" J. Org. Chem. 2014, 79, 1563–1570.
https://doi.org/10.1021/jo4022106

48. Ceban, V.; Putaj, P.; Meazza, M.; Pitak, M. B.; Coles, S. J.; Vesely, J. and Rios, R. "Synergistic catalysis: highly diastereoselective benzoxazole addition to Morita–Baylis–Hillman carbonates" Chem. Commun. 2014, 50, 7447-7450.
https://doi.org/10.1039/C4CC00728J
2013
47. Remeš, M.; Veselý, J.: α-Alkylation of Carbonyl Compounds. In: Stereoselective Organocatalysis: Bond Formation Methodologies and Activation Modes, R. T. Rios (ed.). Wiley, Hoboken, New Jersey, 2013.
https://www.wiley.com/en-ie/9781118604700
Chapter in the book

46. Šimek, M.; Remeš, M.; Veselý, J. and Rios, R. "Enantioselective Organocatalytic Amination of Pyrazolones" Asian J. Org. Chem. 2013, 2, 64–68.
https://doi.org/10.1002/ajoc.201200168

45. Géant, P.-Y.; Urban, M.; Remeš, M.; Císařová, I. and Veselý, J. "Enantioselective Organocatalytic Synthesis of Sulfur-Containing Spirocyclic Compounds" Eur. J. Org. Chem. 2013, 7979–7988.
https://doi.org/10.1002/ejoc.201300931

44. Rios, R.; Hybelbauerová, S.; Císařová, I. and Veselý, J. "First Enantioselective Organocatalytic Addition of Nitromethylphenylsulfone to Enals. Enantioselective Synthesis of Cyclohexenones Bearing 3 Contiguousstereogeniccenters" Curr. Org. Synth. 2013, 10, 467-471.
DOI: 10.2174/1570179411310030008
43. Kamlar, M. and Veselý, J. "Highly enantioselective organocatalytic α-selenylation of aldehydes using hypervalent iodine compounds" Tetrahedron:Asymmetry, 2013, 24, 254–259.
https://doi.org/10.1016/j.tetasy.2013.02.008

42. Fanfrlík, J.; Kolář, M.; Kamlar, M.; Hurný, D.; Ruiz, F. X.; Cousido-Siah, A.; Mitschler, A.; Řezáč, J.; Munusamy, E.; Lepšík, M.; Matějíček, P.; Veselý, J.; Podjarny, A. and Hobza, P. "Modulation of Aldose Reductase Inhibition by Halogen Bond Tuning" ACS Chem. Biol. 2013, 8, 2484–2492.
https://doi.org/10.1021/cb400526n

41. Kamlar, M.; Putaj, P. and Veselý, J. "Organocatalytic alkynylation of densely functionalized monofluorinated derivatives: C(sp3)–C(sp) coupling" Tetrahedron Letters, 2013, 54, 2097–2100.
https://doi.org/10.1016/j.tetlet.2013.02.023

2012
40. Kamlar, M.; Veselý, J. Rios, R.: Diastereoselective Pauson Khand reaction using chiral pool techniques. In: Pauson-Khan reaction: New Trends and Applications, R. T. Rios (ed.). Wiley, VCH, Chichester, UK, 2012. ISBN:978-0-470-97076-8.
https://doi.org/10.1002/9781119941934.ch4
Chapter in the book

39. Remeš, M. and Veselý, J. "Highly Enantioselective Organocatalytic Formation of Functionalized Cyclopentane Derivatives via Tandem Conjugate Addition/α-Alkylation of Enals" Eur. J. Org. Chem. 2012, 3747–3752.
https://doi.org/10.1002/ejoc.201200334

38. Veselý, J. and Rios, R. "Organocatalytic Enantioselective α-Alkylation of Aldehydes" ChemCatChem. 2012, 4, 942 – 953.
https://doi.org/10.1002/cctc.201200072

37. Veselý, J.; Rios, R.: Other transition metal-mediated cyclizations leading to cyclopentenones. In: Pauson-Khan reaction: New Trends and Applications, R. T. Rios (ed.). Wiley, VCH, Chichester, UK, 2012. ISBN:978-0-470-97076-8.
https://doi.org/10.1002/9781119941934.ch10
Chapter in the book

36. Schimer, J.; Cígler, P.; Veselý, J.; Grantz Šašková, K.; Lepšík, M.; Brynda, J.; Řezáčová, P.; Kožíšek, M.; Císařová, I.; Oberwinkler, H.; Kraeusslich, H.-G. and Konvalinka, J. "Structure-Aided Design of Novel Inhibitors of HIV Protease Based on a Benzodiazepine Scaffold" J. Med. Chem. 2012, 55, 10130−10135.
https://doi.org/10.1021/jm301249q

2011
35. Číhalová, S.; Dziedzic, P. and Córdova, A. "Asymmetric Aza-Morita–Baylis–Hillman-Type Reactions: The Highly Enantioselective Reaction between Unmodified α,β- Unsaturated Aldehydes and N-Acylimines by Organo-co-catalysis" Adv. Synth. Catal. 2011, 353, 1096-1108.
https://doi.org/10.1002/adsc.201000951

34. Deiana, L.; Dziedzic, P.; Zhao, G.-L.;Vesely, J.; Ibrahem, I.; Rios, R. Sun, J. and Córdova, A. "Catalytic Asymmetric Aziridination of alpha,beta-Unsaturated Aldehydes" Chem. Eur. J. 2011, 17, 7904 – 7917.
https://doi.org/10.1002/chem.201100042

33. Veselý, J. and Rios, R. "Enantioselective Organocatalytic Synthesis of 5 and 6 Membered Heterocycles" Curr. Org. Chem. 2011, 15, 4046-4082.
https://10.2174/138527211798109213
32. Číhalová, S.; Valero, G.; Schimer, J.; Humpl, M.; Dračínský, M.; Moyano, A.; Rios, R. and Veselý, J. "Highly enantioselective organocatalytic cascade reaction for the synthesis of piperidines and oxazolidines" Tetrahedron, 2011, 67, 8942-8950.
https://doi.org/10.1016/j.tet.2011.08.079

2010
31. Kamlar, M.; Bravo, N.; Alba, A.-N. R.; Hybelbauerová, S.; Císařová,I.; Veselý, J.; Moyano, A.and Rios, R. "Highly Enantioselective Addition of 1-Fluoro-1-nitro(phenylsulfonyl)methane to alpha,beta-Unsaturated Aldehydes"
https://doi.org/10.1002/ejoc.201000851

30. Deiana, L.; Zhao, G.-L.,; Dziedzic, P.; Rios, R.; Veselý, J.; Ekström, J. and Córdova, A. "One-pot highly enantioselective catalytic Mannich-type reactions between aldehydes and stable α-amido sulfones: asymmetric synthesis of β-amino aldehydes and β-amino acids" Tetrahedron Letters, 2010, 51, 234–237.
https://doi.org/10.1016/j.tetlet.2009.10.130

2009
29. Číhalová, S.; Remeš, M.; Císařová, I. and Veselý, J. "ghly Enantioselective Aza-Baylis–Hillman-Type Reaction of α,β-Unsaturated Aldehydes with In Situ Generated N-Boc- and N-Cbz-Imines" Eur. J. Org. Chem. 2009, 6277–6280.
https://doi.org/10.1002/ejoc.200900969

28. Companyó, X.; Hejnová, M.; Kamlar, M.; Veselý, J. ;Moyano, A. and Rios, R. "Highly enantioselective fluoromalonate addition to alpha,beta-unsaturated aldehydes" Tetrahedron Letters, 2009, 50, 5021–5024.
https://doi.org/10.1016/j.tetlet.2009.06.092

27. Valero, G.; Schimer, J.; Cisařová, I.; Veselý, J.; Moyano, A. and Rios "Highly enantioselective organocatalytic synthesis of piperidines. Formal synthesis of (-)-Paroxetine"
Tetrahedron Letters, 2009, 50, 1943–1946.
https://doi.org/10.1016/j.tetlet.2009.02.049

2008
26. Dziedzic, P.; Veselý, J.; Córdova, A. "Catalytic asymmetric synthesis of the docetaxel (Taxotere) side chain: organocatalytic highly enantioselective synthesis of esterification-ready alpha-hydroxy-beta-amino acids" Tetrahedron Letters, 2008, 49, 6631–6634.
https://doi.org/10.1016/j.tetlet.2008.09.038

25. Ibrahem, I.; Rios, R.; Veselý, J.; Zhao, G.-L.; Córdova, A. "Catalytic enantioselective 5-hydroxyisoxazolidine synthesis: An asymmetric entry to beta-amino acids" Synthesis, 2008, 7, 1153-1157.
https://doi.org/10.1055/s-2007-990935
24. Zhao, G.-L.; Veselý, J.; Rios, R.; Ibrahem, I.; Sundén, H.;Córdova, A. "Highly diastereo- and enantioselective catalytic domino thia-Michael/Aldol reactions: Synthesis of benzothiopyrans with three contiguous stereocenters" Adv. Synth. Catal. 2008, 350, 237 – 242.
https://doi.org/10.1002/adsc.200700407

23. Veselý, J.; Rios, R.; Ibrahem, I.; Zhao, G.-L.; Eriksson, L.; Córdova, A."One-pot catalytic asymmetric cascade synthesis of cycloheptane derivatives" Chem. Eur. J. 2008, 14, 2693 – 2698.
https://doi.org/10.1002/chem.200701918

22. Ibrahem, I.; Zhao , G.-L.; Rios, R.; Veselý, J.;Sundén, H.; Dziedzic, P.; Córdova, P. "One-pot organocatalytic domino Michael/alpha-alkylation reactions: direct catalytic enantioselective cyclopropanation and cyclopentanation reactions" Chem. Eur. J. 2008, 14, 7867 – 7879.
https://doi.org/10.1002/chem.200800442

21. Ibrahem, I.; Hammar, P.; Veselý, J.; Rios, R.; Eriksson, L.; Córdova, A. "Organocatalytic asymmetric hydrophosphination of alpha,beta-unsaturated aldehydes: Development, mechanism and DFT calculations" Adv. Synth. Catal. 2008, 350, 1875– 1884.
https://doi.org/10.1002/adsc.200800277

20. Veselý, J.; Zhao, G.-L.; Bartoszewicz, A.; Córdova, A. "Organocatalytic asymmetric nitrocyclopropanation of alpha,beta-unsaturated aldehydes" Tetrahedron Letters, 2008, 49, 4209–4212.
https://doi.org/10.1016/j.tetlet.2008.04.162

19. Zhao, G.-L.; Rios, R.; Veselý, J.; Eriksson, L.; Córdova, A. "Organocatalytic Enantioselective Aminosulfenylation of alpha,beta-Unsaturated Aldehydes" Angew. Chem. Int. Ed. 2008, 47, 8468 –8472.
https://doi.org/10.1002/anie.200802335

18. Zhao, G.-L.; Veselý, J.; Sun, J.; Christensen, K. E.; Bonneau, C.; Córdova, A. "Organocatalytic highly enantioselective conjugate addition of aldehydes to alkylidine malonates" Adv. Synth. Catal. 2008, 350, 657 – 661.
https://doi.org/10.1002/adsc.200700570

17. Veselý, J.; Rios, R.; Córdova, R. "Proline and Lewis base co-catalyzed addition of alpha,beta-unsaturated aldehydes to nitrostyrenes" Tetrahedron Letters, 2008, 49, 1137–1140.
https://doi.org/10.1016/j.tetlet.2007.12.069

16. Turský, M.; Veselý, J.; Tišlerová, I.; Trnka, T.; Ledvina, M. "Synthesis of a new type of D-mannosamine glycosyl donor and acceptor and their use for the preparation of oligosaccharides consisting of D-mannosamine units linked by alpha(1 →4)-glycosidic bonds" Synthesis, 2008, 16, 2610-2616.
DOI: 10.1055/s-2008-1067197
15. Veselý, J.; Rydner, L.; Oscarson, S. "Variant synthetic pathway to glucuronic acid-containing di- and trisaccharide thioglycoside building blocks for continued synthesis of Cryptococcus neoformans capsular polysaccharide structures" Carbohydrate Research 2008, 343, 2200–2208.
https://doi.org/10.1016/j.carres.2007.11.026
2007
14. Rios, R.; Ibrahem, I.; Veselý, J.; Zhao, G. L.; Córdova, A. "A simple one-pot, three-component, catalytic, highly enantioselective isoxazolidine synthesis.“ Tetrahedron Lett. 2007, 48 (32), 5701-5705.
https://doi.org/10.1016/j.tetlet.2007.05.176
13. Rios, R.; Ibrahem, I.; Veselý, J.; Sundén, H.; Córdova, A. "Organocatalytic asymmetric 5-hydroxypyrrolidine synthesis: a highly enantioselective route to 3-substituted proline derivatives.“ Tetrahedron Lett. 2007, 48 (49), 8695-8699.
https://doi.org/10.1016/j.tetlet.2007.10.028
12. Ibrahem, I.; Rios, R.; Veselý, J.; Hammar, P.; Eriksson, L.; Himo, F.; Córdova, A. "Enantioselective organocatalytic hydrophosphination of α,β-unsaturated aldehydes." Angew. Chem. Int. Ed. 2007, 46 (24), 4507-4510.
https://doi.org/10.1002/ange.200700916
11. Veselý, J.; Ibrahem, I.; Rios, R.; Zhao, G. L.; Xu, Y. M.; Córdova, A. "Enantioselective organocatalytic conjugate addition of amines to α,β-unsaturated aldehydes: one-pot asymmetric synthesis of β-amino acids and 1,3-diamines." Tetrahedron Lett. 2007, 48 (12), 2193-2198.
https://doi.org/10.1016/j.tetlet.2007.01.093
10. Ibrahem, I.; Rios, R.; Veselý, J.; Córdova, A. "Organocatalytic asymmetric multi-component C+NC+CC synthesis of highly functionalized pyrrolidine derivatives." Tetrahedron Lett. 2007, 48 (36), 6252-6257.
https://doi.org/10.1016/j.tetlet.2007.07.031
9. Veselý, J.; Dziedzic, P.; Córdova, A. "Aza-Morita-Baylis-Hillman-type reactions: highly enantioselective organocatalytic addition of unmodified α,β-unsaturated aldehydes to N-Boc protected imines." Tetrahedron Lett. 2007, 48 (39), 6900-6904.
https://doi.org/10.1016/j.tetlet.2007.07.154
8. Veselý, J.; Rios, R.; Ibrahem, I.; Córdova, A. "Highly enantioselective organocatalytic addition of unmodified aldehydes to N-Boc protected imines: one-pot asymmetric synthesis of β-amino acids." Tetrahedron Lett. 2007, 48 (3), 421-425.
https://doi.org/10.1016/j.tetlet.2006.11.076
7. Rios, R.; Veselý, J.; Sundén, H.; Ibrahem, I.; Zhao, G. L.; Córdova, A. "One-pot organocatalytic domino Michael/α-alkylation reactions: highly enantioselective synthesis of functionalized cyclopentanones and cyclopentanols." Tetrahedron Lett. 2007, 48 (33), 5835-5839.
https://doi.org/10.1016/j.tetlet.2007.06.070
6. Rios, R.; Sundén, H.; Veselý, J.; Zhao, G. L.; Dziedzic, P.; Córdova, A. "A simple organocatalytic enantioselective cyclopropanation of α,β-unsaturated aldehydes." Adv. Synth. Catal. 2007, 349 (7), 1028-1032.
https://doi.org/10.1002/adsc.200700032
5. Veselý, J.; Ibrahem, I.; Zhao, G. L.; Rios, R.; Córdova, A. Angew. Chem. Int. Ed. "Organocatalytic enantioselective aziridination of α,β-unsaturated aldehydes." 2007, 46 (5), 778-781.
https://doi.org/10.1002/ange.200603810
4. Ibrahem, I.; Rios, R.; Veselý, J.; Zhao, G. L.; Córdova, A. Chem. Commun. "Organocatalytic asymmetric 5-hydroxyisoxazolidine synthesis: A highly enantioselective route to β-amino acids." 2007, 849-851.
https://doi.org/10.1039/B613410F
2006
3. Veselý, J.; Rohlenová, A.; Džoganová, M.; Trnka, T.; Tišlerová, I.; Šaman D.; Ledvina M. "Preparation of ethyl 2-azido-2-deoxy-1-thio-β-d-mannopyranosides, and their rearrangement to 2-S-ethyl-2-thio-β-d-mannopyranosylamines." Synthesis 2006, 669-672.
DOI: 10.1055/s-2006-926297
2004
2. Veselý, J.; Ledvina, M.; Jindřich, J.; Trnka, T.; Šaman, D. "Synthesis of 2-amino- 2-deoxy-β-d-galactopyranosyl-(1®4)-2-amino-2-deoxy-β-d-galactopyranosides: Using various 2-deoxy-2-phthalimido-d-galactopyranosyl donors and acceptors." Coll. Czech. Chem Commun. 2004, 69, 1914-1938.
https://doi.org/10.1135/cccc20041914
2003
1. Veselý, J.; Ledvina, M.; Jindřich, J.; Šaman, D.; Trnka, T. "Improved synthesis of 1,2-trans-acetates and 1,2-trans ethyl 1-thioglycosides derived from 3,4,6-tri-O-acetyl-2-deoxy-phthalimido-d-hexopyranosides." Coll. Czech. Chem Commun. 2003, 68, 1264-1274.
https://doi.org/10.1135/cccc20031264