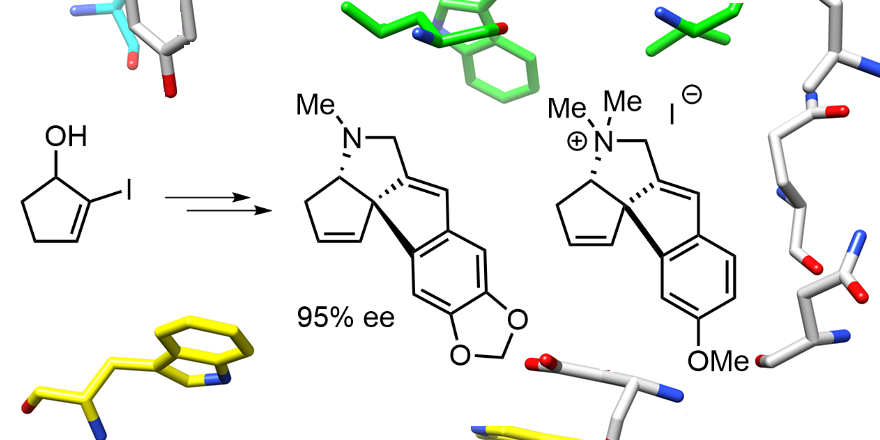
(15) Oxidative Cleavage and Ring Reconstruction in the Synthesis of Amaryllidaceae Alkaloid Analogues.
Jansa, P.; Císařová, I.; Matoušová, E. Eur. J. Org. Chem. 2024, 27, e202301021.
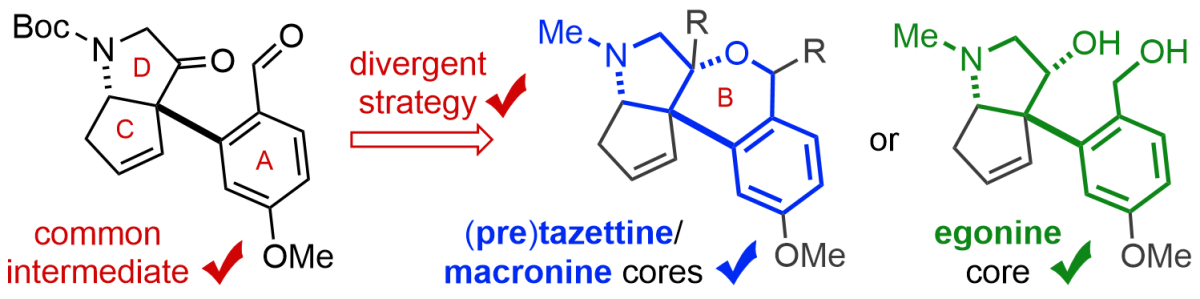
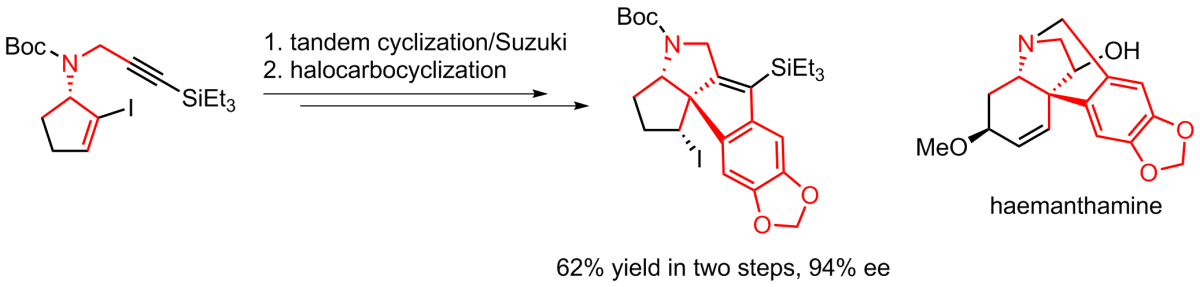
(14) Tandem Alkyne Carbopalladation/Suzuki Cross-Coupling Reaction in the Synthesis of Heterocyclic Compounds.
Jagtap, P. R.; Matoušová, E. In Targets in Heterocyclic Systems - Chemistry and Properties; Italian Chemical Society: Rome, 2022; Vol. 26, pp 1–17.
ISBN: 978-8886208161
(13) Synthesis and Cholinesterase Inhibitory Activity Study of Amaryllidaceae Alkaloid Analogues with N-Methyl Substitution.
Jansa, P.; Barvík, I.; Hulcová, D.; Matoušová, E. Org. Biomol. Chem. 2022, 20, 3960–3966.
(12) Synthesis of Fused 1,2-Naphthoquinones with Cytotoxic Activity Using a One-Pot Three-Step Reaction.
Nechaev, A. A.; Jagtap, P. R.; Bažíková, E.; Neumannová, J.; Císařová, I.; Matoušová, E. Org. Biomol. Chem. 2021, 19, 3434–3440.
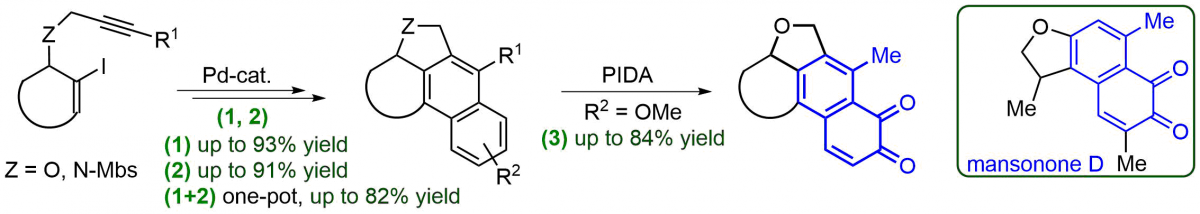
(11) Enantioselective Synthesis of All-Carbon Quaternary Centers Structurally Related to Amaryllidaceae Alkaloids.
Mikušek, J.; Jansa, P.; Jagtap, P. R.; Vašíček, T.; Císařová, I.; Matoušová, E. Chem. Eur. J. 2018, 24, 10069–10072.
Post-doctoral Studies:
(10) The Palladium-Catalyzed Intramolecular Alder-Ene Reactions of O- and N-Linked 1,6-Enynes Incorporating Triethylsilyl Capping Groups.
Nugent, J.; Matoušová, E.; Banwell, M. G.; Willis, A. C. J. Org. Chem. 2017, 82, 12569–12589.
(9) Enantioselective Synthesis of the Unsaturated Fragment of Callyspongiolide.
Matoušová, E.; Koukal, P.; Formánek, B.; Kotora, M. Org. Lett. 2016, 18, 5656–5659.
(8) [2+2+2]-Cyclotrimerization of 1-Cyclopropyl-1,6-diynes with Alkynes: Formation of Cyclopropylarenes.
Matoušová, E.; Gyepes, R.; Císařová, I.; Kotora, M. Adv. Synth. Catal. 2016, 358, 254–267.
(7) A Total Synthesis of Galanthamine Involving De Novo Construction of the Aromatic C-Ring.
Nugent, J.; Matoušová, E.; Banwell, M. G. Eur. J. Org. Chem. 2015, 2015, 3771–3778.
(6) The Palladium-Catalysed Intramolecular Alder-ene (IMAE) Reactions of Certain Heteroatom-Linked 1,6-Enynes: The Formation of Hexahydro-Indoles and -Benzofurans.
Crisp, A. L.; Li, J.; Lan, P.; Nugent, J.; Matoušová, E.; Banwell, M. G. Aust. J. Chem. 2015, 68, 1183–1189.
DOI: 10.1071/CH15340
(5) Devising New Syntheses of the Alkaloid Galanthamine, a Potent and Clinically Deployed Inhibitor of Acetylcholine Esterase.
Banwell, M. G.; Buckler, J.; Jackson, C. J.; Lan, P.; Ma, X.; Matoušová, E.; Nugent, J. In Strategies and Tactics in Organic Synthesis; Academic Press: Oxford, 2015; Vol. 11, pp 29–50.
ISBN: 978-0-08-100023-6
(4) A Chemoenzymatic Total Synthesis of the Protoilludane Aryl Ester (+)-Armillarivin.
Schwartz, B. D.; Matoušová, E.; White, R.; Banwell, M. G.; Willis, A. C. Org. Lett. 2013, 15, 1934–1937.
PhD Studies:
(3) Substrate Control in the Gold(I)-Catalyzed Cyclization of β-Propargylamino Acrylic Esters and Further Transformations of the Resultant Dihydropyridines.
Mikušek, J.; Matouš, P.; Matoušová, E.; Janoušek, M.; Kuneš, J.; Pour, M. Adv. Synth. Catal. 2016, 358, 2912–2922.
(2) TFP as a ligand in Au(I)-catalyzed dihydropyran synthesis. Unprecedented rearrangement of dihydropyrans into cyclopentenones.
Matoušová, E.; Růžička, A.; Kuneš, J.; Králová, J.; Pour, M. Chem. Commun. 2011, 47, 9390–9392.
(1) Synthesis and biological activity of desmethoxy analogues of coruscanone A.
Tichotová, L.; Matoušová, E.; Špulák, M.; Kuneš, J.; Votruba, I.; Buchta, V.; Pour, M. Bioorg. Med. Chem. Lett. 2011, 21, 6062–6066.