We are currently working on the following research projects:
Bringing Green Chemical Production Forward in Central Europe
We are involved in an international Interreg Central Europe project, co-funded by the European Regional Development Fund.

Using levoglucosenone as a starting material in synthesis of potentially bioactive compounds
Cellulosic-based material is the most abundant and renewable form of organic matter on the planet, and levoglucosenone, being collulosic-based and moreover a chiral compound, has a potential to become a valued chemical building block in the future. As such, discovering new applications of this interesting substance in synthesis is likely to be important.
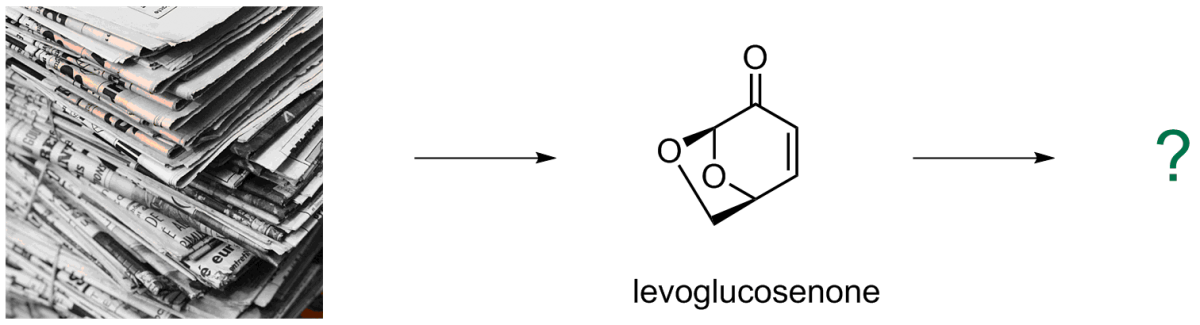
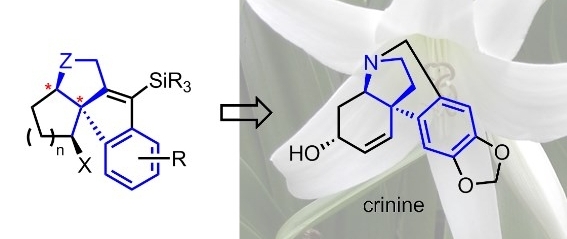
Enantioselective construction of all-carbon quaternary centres
All-carbon quaternary centres occur widely in natural products and pharmaceutically important compounds. Their preparation in an enantioselective fashion, however, still represents one of the greatest challenges in synthetic organic chemistry. This project deals with asymmetric formation of quaternary centres and its possible application in the synthesis of biologically active compounds, such as crinine- and haemanthamine-type Amaryllidaceae alkaloids and other structurally related substances. Our main focus is on using tandem cyclisation/Suzuki cross-coupling and subsequent halocarbocyclisation reaction sequence as a new method to achieve this goal.
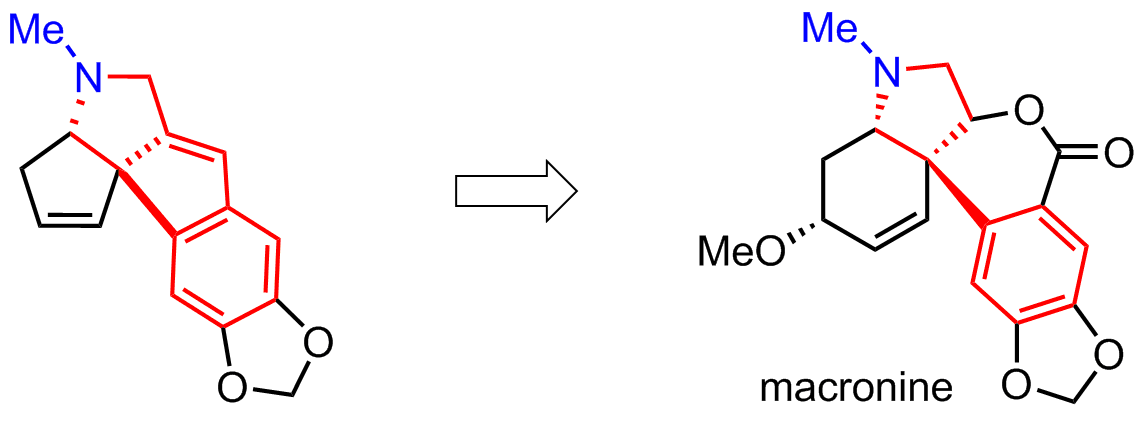
Synthesis of alkaloid-like polycyclic compounds
One of the modifications of our method is to use an N-methylated starting materials. It is useful because methylated nitrogen is present in some alkaloids, such as macronine, and in this approach there is no need for protecting groups on nitrogen atom. We are currently optimising oxidation reactions of the two double bonds in our product.