An innovative approach to planar chiral substances
Dr. Vojtěch Dočekal and Prof. Jan Veselý from the Department of Organic Chemistry, Faculty of Science, Charles University described a new highly efficient method for the preparation of planar chiral compounds.
The method based on organocatalytic desymmetrization was published in the prestigious journal Nature Communications. Congratulations!

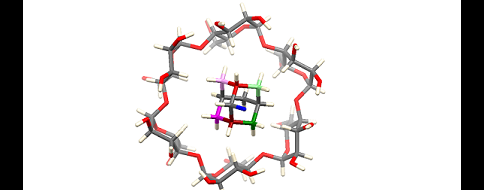
Planar chiral [2.2]paracyclophanes contain two suitably substituted benzene nuclei linked at the para positions by ethylene bridges. Such compounds have a wide range of applications either as ligands or catalysts in synthetic chemistry, but also in materials science or medicine. Despite the wide range of applications, only a limited number of studies have been described for their preparation in enantiomerically pure form.
The methodology developed in the asymmetric synthesis group is based on the highly efficient desymmetrization of prochiral [2.2]paracyclophanes substituted with two formyl groups in suitable positions. In a key transformation, one of the prochiral aldehyde groups is selectively transformed to an ester, leading to the formation of a planar chiral product using asymmetric synthesis methods. Methodologies using catalysis by low molecular weight chiral catalysts derived from natural amino acids, more specifically using so-called N-heterocyclic carbenes, have proven to be highly effective for such asymmetric esterification.
In addition to the optimization of the methodology, a study of the scope of application of the developed transformation, or a study of the synthetic applicability of the chiral products, the publication includes a detailed experimental mechanistic study that reveals interesting differences in the reactivity of the starting diformyl[2.2]paracyclophanes.