38. C–H amination of enolizable and nonenolizable ketones

Holovko-Kamoshenkova, O.; Tošner, Z.; Císařová, I.; Hrdina, R. Org. Biomol. Chem. 2025, DOI: 10.1039/D5OB00009B.
37. O původu homochirality na Zemi
Hrdina, R. Vesmír 2024, 488-491.
36. A Meta-Analysis of the Mortality and the Prevalence of Burn Complications in Western Populations
Foppiani, J. A.; Weidman, A. ; Hernandez Alvarez, A.; Valentine, L.; Bustos, V. P.; Galinaud, C.; Hrdina, R.; Hrdina, R.; Musil, Z.; Lee, B. T.; Lin, S. J. J Burn Care Res 2024, DOI: 10.1093/jbcr/irae064.
35. Spatial arrangements of cyclodextrin host–guest complexes in solution studied by 13C NMR and molecular modelling
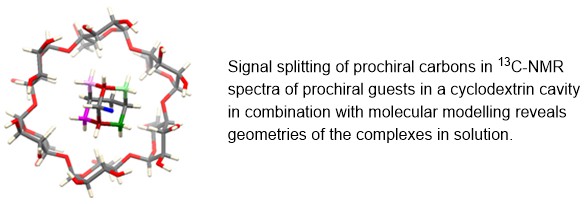
Lebedinskiy, K.; Barvík, I.; Tošner, Z.; Císařová, I.; Jindřich, J.*; Hrdina, R.* Beilstein J. Org. Chem. 2024, 20, 331-335; https://doi.org/10.3762/bjoc.20.33
34. Synthesis of 1,2-disubstituted adamantane derivatives by construction of the adamantane framework
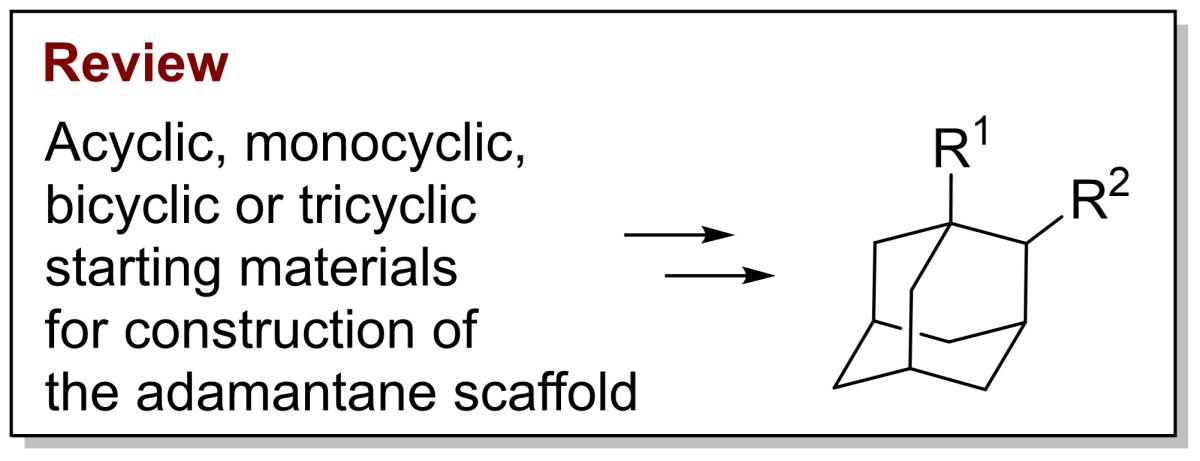
Todd, M.; Hrdina R. Molecules 2023, 28, 7636, https://doi.org/10.3390/molecules28227636.
33. En Route to Local Glioblastoma Treatment with Temozolomide Doped Hyaluronan Fibres: Formulation and In Vitro Cell Studies
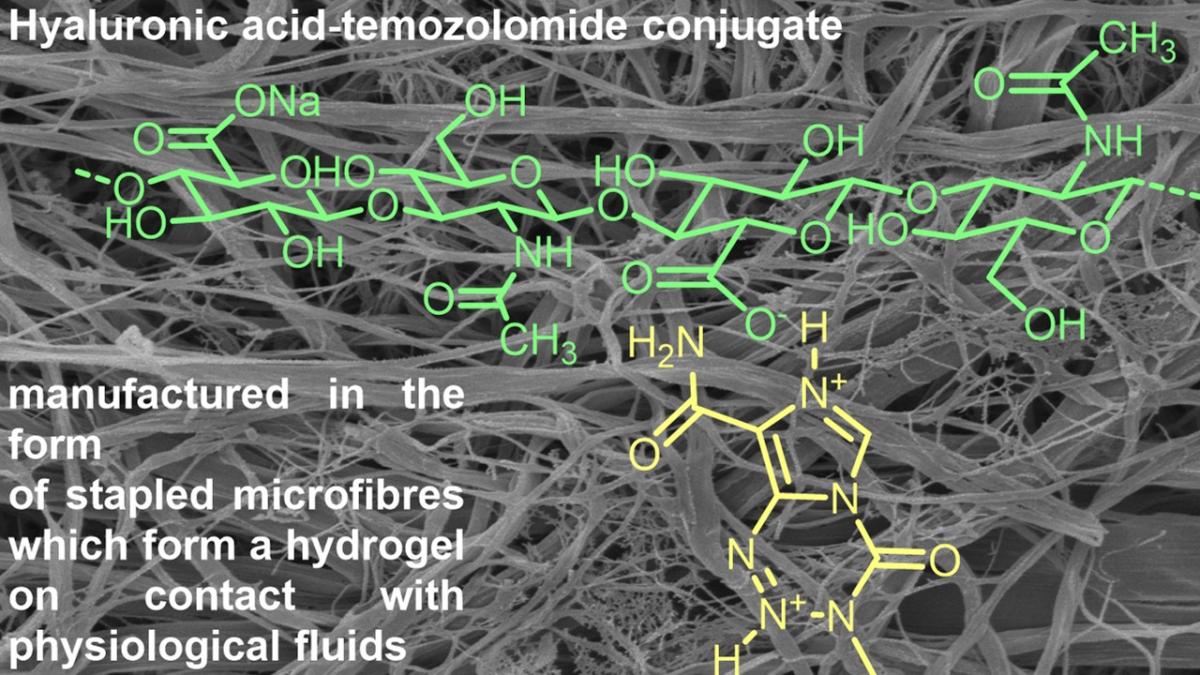
Belhajová, M.; Vícha, A.; Burgert, L.; Brožková, I.; Michalíčková, L.; Hrdina, R.; Moravec, T.; Netuka, D.; Musil, Z.; Hrdina, R. RSC Med. Chem. 2023, DOI: 10.1039/D3MD00261F.
33-CP. Cover picture in RSC Med. Chem. RSC Med. Chem., 2023, 14, 1584-1584.
32. An Efficient Catalyst-Free Direct Approach to 5-Polyfluoroalkyl-1,2,4-triazole-3-thiones
Holovko-Kamoshenkova, O. M.; Slivka, M. V.; Hrdina, R.; Baumer, V. N.; Korol, N. I.; Sokolenko, L. V.; Lendel V. G. Synthesis 2022, DOI: 10.1055/s-0042-1751401.
31. Annulated carbamates are precursors for the ring contraction of the adamantane framework

Hrdina, R.; Holovko-Kamoshenkova, O. M.; Císařová, I.; Koucký, F.; Machalický, O. RSC Advances 2022, DOI: 10.1039/d2ra06402b.
30. Synthesis of Noradamantane Derivatives by Ring-Contraction of the Adamantane Framework
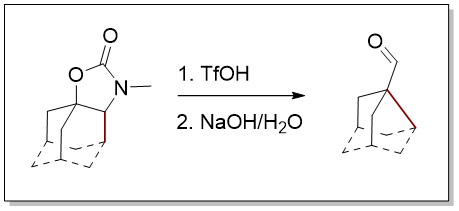
Zonker, B.; Becker, J.; Hrdina, R. Org. Biomol. Chem. 2021, DOI: 10.1039/D1OB00471A.
29. Overview of Dirhodium(II,II) Paddlewheel Complexes
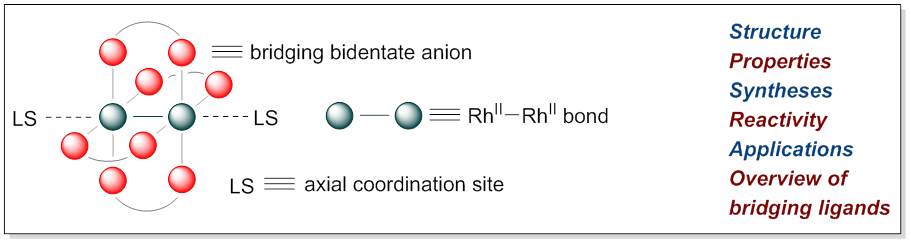
Hrdina, R. Eur. J. Inorg. Chem. 2021, DOI:10.1002/ejic.202000955.
28. [1,2]-Rearrangement of Iminium Salts Provides Access to Heterocycles with Adamantane Scaffold
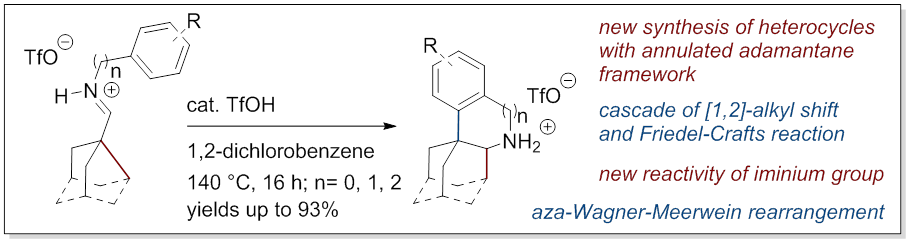
Zonker, B.; Duman, E.; Hausmann, H.; Becker, J.; Hrdina, R. Org. Biomol. Chem. 2020, DOI: 10.1039/D0OB01156H.
27. Stereoselective deconjugation of macrocyclic alpha,beta-unsaturated esters by sequential amidation and olefin transposition:
application to enantioselective phase-transfer catalysis
Homberg, A.; Hrdina, R.; Vishe, M.; Guénée, L.; Lacour, J. Org. Biomol. Chem. 2019, DOI: 10.1039/c9ob01355e.
26. Diamondoid Amino Acid-based Peptide Kinase A Inhibitor Analogues
Müller, J.; Kirschner, R. A.; Berndt, J.-P.; Wulsdorf, T.; Metz, A.; Hrdina, R.; Schreiner, P. R.; Geyer, A.; Klebe, G. Chem. Med. Chem. 2019, DOI: 10.1002/cmdc.201800779.
25. Site-Selective Nitrenoid Insertions Utilizing Postfunctionalized Bifunctional Rhodium(II) Catalysts
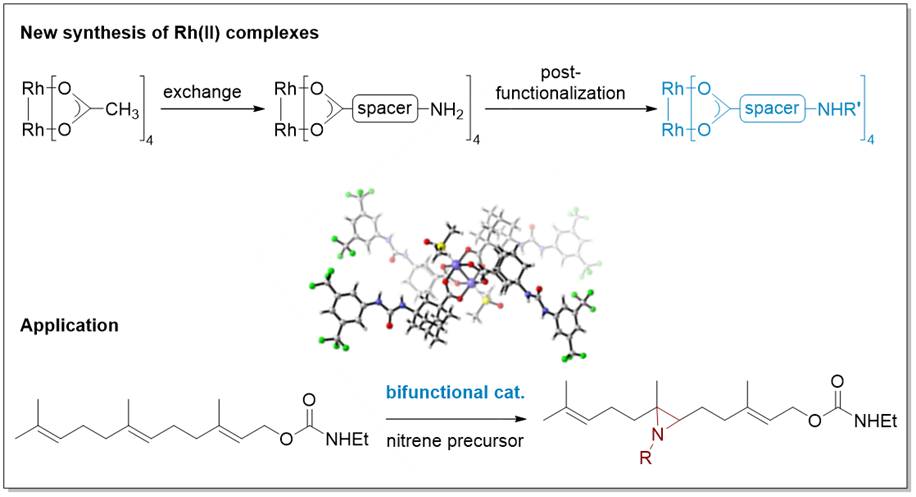
Berndt, J.-P.; Radchenko, Y.; Becker, J.; Logemann, C.; Bhandari, D. R.; Hrdina, R.* and Schreiner, P. R.* Chem. Sci. 2019, DOI: 10.1039/C8SC05733H.
24. Azido-Adamantyl Tin Sulfide Clusters for Bioconjugation
Berndt, J.-P.; Engel, A.; Hrdina, R.; Dehnen, S.; Schreiner, P. R. Organometallics 2019, DOI: 10.1021/acs.organomet.8b00734.
23. Directed C–H Functionalization of the Adamantane Framework
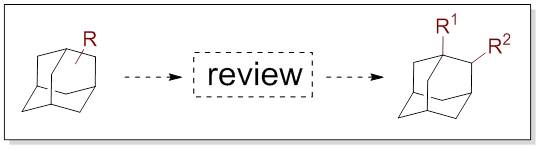
Hrdina, R. Synthesis 2019, DOI: 10.1055/s-0037-1610321.
22. Directed C−H Bond Oxidation of Bridged Cycloalkanes Catalyzed by Palladium(II) Acetate
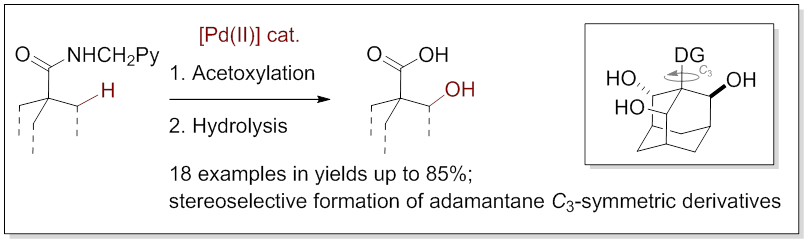
Larrosa, M.; Zonker, B.; Volkmann, J.; Wech, F.; Logemann, C.; Hausmann, H.; Hrdina, R. Chem. Eur. J. 2018, DOI: 10.1002/chem.201800550.
21. Triflic Acid Promoted Decarboxylation of Adamantane-Oxazolidine-2-one: Access to Chiral Amines and Heterocycles
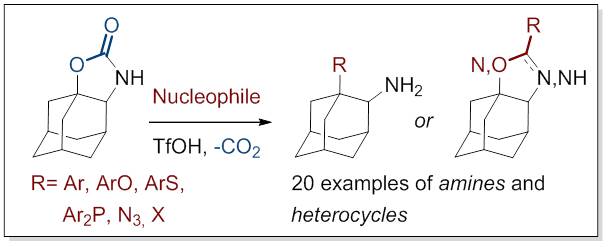
Hrdina, R; Larrosa, M.; Logemann, C. J. Org. Chem. 2017, DOI: 10.1021/acs.joc.7b00711.
20. Peptide-Functionalized Organotin Sulfide Clusters
Rinn, N.; Berndt, J.-P.; Kreher, A.; Hrdina, R.; Reinmuth, M.; Schreiner, P.R.; Dehnen, S. Organometallics, 2016, DOI: 10.1021/acs.organomet.6b00561.
19. C−H bond arylation of diamondoids catalyzed by palladium(II) acetate
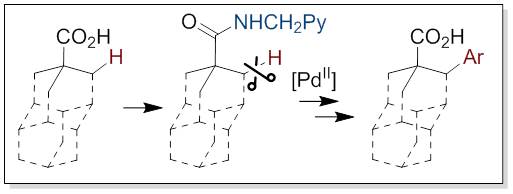
Larrosa, M.; Heiles, S.; Becker, J.; Spengler, B.; Hrdina, R. Adv. Synth. Catal. 2016, DOI: 10.1002/adsc.201600156.
18. Intramolecular C-H amination provides direct access to 1,2-disubstituted diamondoids
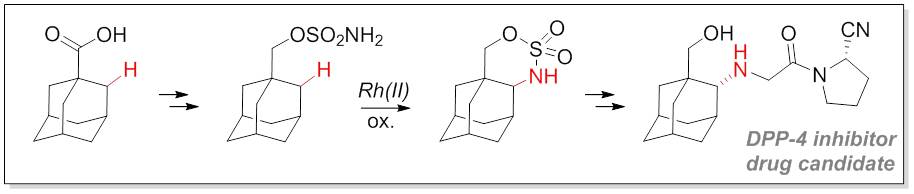
Hrdina, R.; Metz, F. M.; Larrosa, M.; Berndt, J.-P.; Zhygadlo, Y. Y.; Becker, S.; Becker, J. Eur. J. Org. Chem. 2015, DOI: 10.1002/ejoc.201500691.
17. Vishe, M.; Hrdina, R.; Poblador-Bahamonde, A. I.; Besnard, C.; Guénée, L.; Bürgi, T.; Lacour, J. Chem. Sci. 2015, 6, 4923.
16. Vishe, M.; Hrdina, R.; Guénée, L.; Besnard, C.; Lacour, J. Adv. Synth. Catal. 2013, 355, 3161.
15. Hrdina, R.; Guénée, L.; Moraleda, D.; Lacour, J. Organometallics 2013, 32, 473.
14. Müller, C. E.; Zell, D. M.; Hrdina, R.; Wende, R. C.; Wanka, L.; Schuler, S. M. M.; Schreiner P. R. J. Org. Chem. 2013, 78, 8465.
13. Hrdina, R.; Müller, C. E.; Wende, R. C.; Wanka, L.; Schreiner, P. R. Chem. Commun. 2012, 48, 2498.
12. Müller, C. E.; Hrdina, R.; Wende, R. C.; Schreiner, P. R. Chem. Eur. J. 2011, 17, 6309.
11. Hrdina, R.; Müller, C. E.; Wende, R. C.; Lippert, K. M.; Benassi, M.; Spengler, B.; Schreiner, P. R. J. Am. Chem. Soc. 2011, 133, 7624.
10. Hrdina, R.; Müller, C. E.; Schreiner, P. R. Chem. Commun. 2010, 46, 2689. (highlighted in Synfacts 2010, 5, 594.)
9. Vlašaná, K.; Hrdina, R.; Valterová, I.; Kotora, M. Eur. J. Org. Chem. 2010, 7040.
8. Kadlčíková, A.; Hrdina, R.; Valterová, I.; Kotora, M. Adv. Synth. Catal. 2009, 351, 1279.
7. Hrdina, R.; Opekar, F.; Roithová, J.; Kotora, M. Chem. Commun. 2009, 2314.
6. Hrdina, R.; Boyd, T.; Valterová, I.; Hodačová, J.; Kotora, M. Synlett 2008, 3141.
5. Hrdina, R.; Dračínský, M.; Valterová, I.; Hodačová, J.; Císařová, I.; Kotora, M. Adv. Synth. Catal. 2008, 350, 1449.
4. Hrdina, R.; Valterová, I.; Hodačová, J.; Kotora, M. Adv. Synth. Catal. 2007, 349, 822.
3. Hrdina, R.; Kadlčíková, A.; Valterová, I.; Hodačová, J.; Kotora, M. Tetrahedron: Asymmetry 2006, 17, 3185.
2. Hrdina, R.; Stará, I. G.; Dufková, L.; Scott, M.; Císařová, I.; Kotora, M. Tetrahedron 2006, 62, 968.
1. Belokon, Y. N.; Bespalova, N.; Churkina, T. D.; Císařová, I.; Ezernitskaya, M. G.; Harutyunyan, S. R.; Hrdina, R.; Kagan, H. B.; Kočovský, P.; Kochetkov, K. K.; Larionov O. V.; Lyssenko, K. A.; North, M.; Polášek M.; Peregudov, A. S.;. Prisyazhnyuk, V. V.; Vyskočil, Š. J. Am. Chem. Soc. 2003, 125, 12860.


